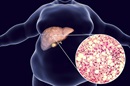
Resmetirom, a thyroid hormone receptor-beta (THR-β) agonist, received accelerated FDA approval to treat nonalcoholic steatohepatitis (NASH) in patients with moderate to advanced liver fibrosis. In NASH, hepatic THRs are impaired, leading to fatty acid accumulation and fibrosis. Resmetirom stimulates THR receptors, promoting triglyceride metabolism and reducing hepatic adiposity. The effects of resmetirom on NASH-induced liver disease were evaluated in the ongoing MAESTRO-NASH trial (N=966), where 52-week interim results showed that it was superior to placebo for the two primary outcomes, NASH resolution (30% vs 9.7%) and fibrosis improvement (26% vs 14.2%). The full trial is planned for 54 months and will include the following endpoints: mortality, hepatic decompensation (e.g., ascites, hepatic encephalopathy, variceal bleeding), and liver transplant.
Resmetirom is given as a once-daily tablet with weight-based dosing. Common side effects are diarrhea (33%), nausea (15%), pruritus (10%), vomiting (8%), and constipation (8%). Notable drug interactions requiring dose adjustments include certain statins and CYP2C8 inhibitors.
In the past, I've been reluctant to pursue extensive NASH workups since the only real treatment was weight loss. Now, I have to give it more consideration. The main challenge is identifying patients who will benefit the most from resmetirom, as it is expensive, and the majority of NASH patients do not progress to cirrhosis. For primary care doctors, selecting appropriate candidates may follow the following sequence: (1) Calculate a
FIB-4 score; (2) If FIB-4 is > 1.3, order an
ELF test; (3) If ELF is > 7.7, order a liver biopsy; (4) if F2 or F3 fibrosis is present, start resmetirom. Given its projected cost of $47,400 per year, it's likely insurance companies will require prior authorization that includes proof of liver fibrosis.